Huperzine A: Complete Evidence-Based Review, Dosing & Cycling Guide
Quick Summary: Huperzine A
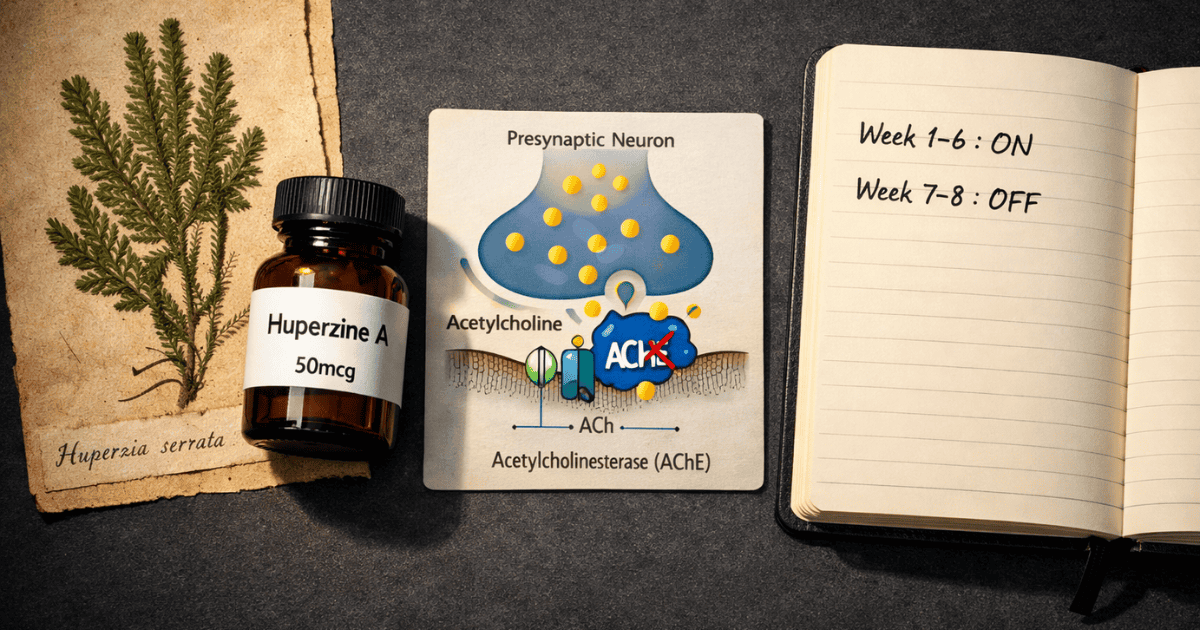
- Primary mechanism: Reversible acetylcholinesterase (AChE) inhibition — prevents enzymatic breakdown of acetylcholine in synaptic clefts
- Secondary mechanisms: Weak NMDA receptor antagonism, antioxidant activity, BDNF upregulation, neuroprotection against glutamate excitotoxicity
- Clinical evidence: Strong in Alzheimer’s disease (20 RCTs, 1,823 participants); limited in healthy, cognitively intact populations
- Standard dose (cognitive enhancement): 50–100 mcg once daily
- Half-life: ~10–14 hours — single daily dose sufficient; daily accumulation risk is real
- Cycling is required: 4–8 weeks on, 1–2 weeks off; continuous use not recommended
- Critical interactions: Contraindicated alongside prescription cholinesterase inhibitors (donepezil, rivastigmine, galantamine); additive effects with beta-blockers
- Stack role: Completes the cholinergic triad — Alpha-GPC builds ACh, Phosphatidylserine releases it, Huperzine A protects it from enzymatic breakdown
What Is Huperzine A? The Compound That Completes the Cholinergic Architecture
If you have spent time researching cognitive enhancement, you have almost certainly encountered recommendations for Alpha-GPC, the most bioavailable choline donor available. You may have added Phosphatidylserine, which supports acetylcholine release at the presynaptic membrane. Both are solid choices — and the NeuroEdge Formula research library covers them in detail.
But here is the gap that most nootropic protocols never address: neither Alpha-GPC nor Phosphatidylserine does anything to prevent the acetylcholine you have just produced from being immediately destroyed.
That is the job of Huperzine A.
Huperzine A is a sesquiterpene alkaloid first isolated in 1983 from Huperzia serrata, a Chinese club moss plant used in traditional medicine for centuries. Chinese scientists at the Shanghai Institute of Materia Medica identified it as one of the most selective, reversible acetylcholinesterase inhibitors ever characterized — more selective than the pharmaceutical AChE inhibitors that would later become standard Alzheimer’s treatment, and with a substantially wider margin of safety.
In China, it has been approved as a prescription drug for Alzheimer’s disease since the early 1990s, with over 100,000 patients treated in clinical programs without reported serious adverse events. In the United States, it is classified as a dietary supplement by the FDA and is widely available, typically marketed as a memory and concentration enhancer.
The distinction matters: this is not a mild botanical adaptogen. Huperzine A is pharmacologically active at doses measured in micrograms. Its mechanism is shared with donepezil, rivastigmine, and galantamine — three FDA-approved drugs for Alzheimer’s disease. Understanding what it does at the molecular level is not optional background; it is the foundation for using it appropriately and safely.
The Cholinergic Problem: Why Acetylcholine Breaks Down So Fast
Acetylcholine (ACh) is the primary neurotransmitter of the cholinergic system — the network most directly associated with memory encoding, attention, and learning. When a neuron fires and releases ACh into the synaptic cleft, that signal must be terminated quickly and precisely. The enzyme responsible for this termination is acetylcholinesterase (AChE).
AChE is extraordinarily efficient. It hydrolyzes acetylcholine into choline and acetate within milliseconds of release, ending the signal at the synapse. The choline component is partially recycled, but the signal itself is gone.
In a healthy, well-nourished brain, this rapid clearance is appropriate. The system works as designed. But in conditions of cholinergic insufficiency — which research suggests increases with normal aging even in healthy populations — the rate of ACh breakdown may outpace the rate of synthesis, creating a functional deficit in cholinergic transmission even when choline substrate is theoretically adequate.
This is where the three-compound cholinergic architecture comes together:
- Alpha-GPC provides the choline substrate for acetylcholine synthesis — building the molecule upstream
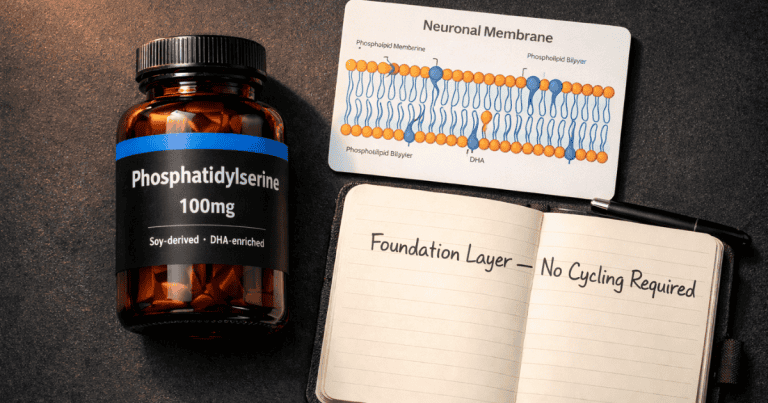
- Phosphatidylserine supports the presynaptic membrane integrity and acetylcholine release dynamics — facilitating the signal
- Huperzine A inhibits the enzyme that destroys ACh — extending the signal’s duration in the synapse
The pharmacokinetics align usefully. According to Li et al. (2007), Alpha-GPC reaches peak plasma concentration at approximately 1 hour after dosing, while Huperzine A peaks at roughly 58 minutes but maintains meaningful AChE inhibition for 10–14 hours due to its long β-elimination half-life. The result is a cholinergic environment where acetylcholine is being supplied and its degradation is being slowed simultaneously — a mechanistic pairing with stronger theoretical rationale than either compound alone.
Mechanisms of Action: Beyond Simple AChE Inhibition
Huperzine A is often summarized as “an acetylcholinesterase inhibitor” — which is accurate but incomplete. It works through at least four distinct mechanisms, each contributing to its cognitive and neuroprotective profile.
Primary Mechanism: Reversible, Selective AChE Inhibition
Huperzine A binds to the active site of acetylcholinesterase with an IC50 of approximately 82 nM — an exceptionally high binding affinity. Critically, this inhibition is reversible: unlike organophosphate compounds, which bind irreversibly and can cause fatal cholinergic crisis, Huperzine A slowly dissociates from the enzyme, allowing normal AChE activity to resume when the compound clears.
Its selectivity for AChE over butyrylcholinesterase (BuChE) is also notably higher than pharmaceutical AChE inhibitors including donepezil and galantamine. BuChE inhibition is associated with peripheral cholinergic side effects including gastrointestinal disturbance, bradycardia, and excessive secretions — so Huperzine A’s AChE selectivity contributes to its comparatively favorable side effect profile.
The X-ray crystal structure of the Huperzine A–AChE complex has been fully characterized (PDB: 1VOT), confirming it fits precisely into the enzyme’s active gorge and providing the molecular explanation for its remarkable potency at very low doses.
Secondary Mechanism: NMDA Receptor Modulation
Huperzine A is also a weak NMDA receptor antagonist, with an IC50 in the range of 65,000–82,000 nM — far lower affinity than its AChE binding. At typical human dosing ranges (50–200 mcg), meaningful NMDA blockade is pharmacologically unlikely. However, this activity may contribute to neuroprotection under conditions of glutamate excitotoxicity — the neurotoxic cascade that occurs when receptor overstimulation causes excessive calcium influx and neuronal death.
This mechanism is particularly relevant in discussions of Alzheimer’s disease, where glutamate excitotoxicity is a recognized contributor to neurodegeneration. Preliminary evidence suggests that combination therapy with memantine and Huperzine A may produce additive benefits, though this has not been validated in large-scale trials.
Antioxidant and Mitochondrial Protection
In vitro and animal studies have documented Huperzine A’s ability to reduce reactive oxygen species, protect against hydrogen peroxide-induced cytotoxicity, and preserve mitochondrial membrane integrity in neuronal models exposed to beta-amyloid toxicity. It upregulates anti-apoptotic proteins including Bcl-2 while downregulating pro-apoptotic proteins Bax, P53, and caspase-3.
Whether these effects translate meaningfully to human cognitive function at supplement doses remains uncertain. The concentrations used in cell culture studies often exceed what is achievable in human brain tissue through oral supplementation. These mechanisms are better understood as contributing to Huperzine A’s broader neuroprotective profile than as primary drivers of its acute cognitive effects.
BDNF Upregulation
Emerging preclinical research suggests Huperzine A may upregulate brain-derived neurotrophic factor (BDNF) — approximately a 2-fold increase in preclinical models. BDNF is a key signaling protein for neuroplasticity, synaptic growth, and long-term potentiation. If confirmed at relevant human doses, this would place Huperzine A alongside Lion’s Mane (which stimulates NGF) as a compound supporting both immediate neurotransmitter optimization and longer-term structural brain health. Current evidence for this mechanism in humans remains preliminary.
Clinical Evidence: What the Research Actually Shows
The evidence base for Huperzine A is substantial by nootropic standards — but it comes with important caveats that most competitor content omits. Understanding where the evidence is strong, where it is weak, and why the distinction matters is central to forming an accurate picture.
Evidence in Alzheimer’s Disease: Robust but Methodologically Limited
The most comprehensive independent analysis is a 2013 PLOS ONE systematic review and meta-analysis (Yang et al.) that identified 20 randomized clinical trials encompassing 1,823 participants, with treatment durations ranging from 8 to 36 weeks at daily doses of 0.2–0.8 mg. Compared to placebo, Huperzine A showed statistically significant improvements in cognitive function as measured by MMSE at 8, 12, and 16 weeks, in the Hasegawa Dementia Scale and Wechsler Memory Scale at 8 and 12 weeks, and in Activities of Daily Living at 6, 12, and 16 weeks.
A separate 2014 meta-analysis (Xing et al.) covering 8 Alzheimer’s trials (733 participants) and 2 vascular dementia trials (92 participants) confirmed these findings, with longer treatment durations consistently associated with larger effect sizes in AD patients.
However — and this caveat is critical — the 2013 PLOS ONE authors explicitly acknowledged that methodological quality of most included trials was rated at high risk of bias. The majority of trials originated from Chinese research groups, many have not been independently replicated, and an asymmetrical funnel plot in the analysis suggested possible publication bias toward positive findings. A 2008 Cochrane review (Li et al.) reached a more conservative conclusion, characterizing the evidence as inconclusive due to small sample sizes and limited methodological quality.
The one large-scale U.S. trial — a Phase II RCT conducted through the Alzheimer’s Disease Cooperative Study (Rafii et al., 2011; n=210) — found no significant improvement on the ADAS-Cog primary outcome measure, though a positive signal appeared on MMSE. This negative result on the gold-standard cognitive outcome measure in a rigorously designed Western trial is the honest counterweight to the predominantly positive Chinese literature.
Evidence in Healthy Populations: The Honest Gap
Here is where most Huperzine A marketing quietly switches from evidence to extrapolation without signaling the transition: the evidence base in cognitively healthy adults is genuinely thin.
A 1999 Chinese study evaluated Huperzine A supplementation in 68 junior high school students with memory complaints. After four weeks, the Huperzine A group showed superior performance on memory tests compared to placebo — this is frequently cited as evidence for cognitive enhancement in healthy young people. The study is real, but a single small trial in adolescents with self-reported memory complaints is a narrow foundation for broad claims about healthy adult cognitive enhancement.
A 2021 randomized crossover trial (Wessinger et al.) evaluated 200 mcg Huperzine A on cognitive function and perceived effort during exercise in healthy adults. The trial found no significant improvement in standard cognitive measures, though subjective perceived effort was somewhat lower in the Huperzine A condition — a modestly interesting finding that does not support the compound’s reputation for dramatic memory enhancement in healthy populations.
A 2016 systematic review (Zheng et al.) documented adjunctive Huperzine A benefits for cognitive deficits in schizophrenia across 3 trials (238 patients) — adding to the evidence of cholinergic enhancement effects, but again in a clinical population, not healthy adults.
The honest summary: Huperzine A’s mechanism is pharmacologically confirmed, its effects in dementia populations are relatively well-documented despite methodological limitations, and its effects in healthy, cognitively intact adults are plausible but substantially under-researched. Those using it for cognitive enhancement are extrapolating from a mechanistic rationale and population-specific evidence — which is not unusual in nootropics, but is worth stating clearly.
Dosing Protocol: The Critical Difference Between Therapeutic and Enhancement Ranges
Huperzine A dosing requires precision that most other nootropics do not, for two reasons: it is active at exceptionally low doses, and its long half-life creates accumulation risk with daily use.
Standard Doses for Cognitive Enhancement
For healthy adults, the standard dose range is 50–100 mcg once daily. This is substantially lower than the doses used in Alzheimer’s disease clinical trials, which typically ranged from 200–400 mcg twice daily (400–800 mcg total daily). The Alzheimer’s disease dose is calibrated to compensate for severe cholinergic deficit; a healthy brain does not require — and should not receive — the same degree of AChE inhibition.
Most experienced nootropic users report that 50 mcg is an effective starting point, with 100 mcg representing a reasonable upper boundary for standard use. Doses above 200 mcg in healthy individuals increase cholinergic side effect risk without proportionate cognitive benefit.
Confirm the unit carefully when purchasing: some products use mg labeling. 0.05 mg = 50 mcg; 0.1 mg = 100 mcg. The distinction is not trivial.
The Cycling Requirement: Why It Is Non-Negotiable
This is the aspect of Huperzine A use that most users get wrong — often because supplement products actively discourage thinking about it.
Based on human pharmacokinetic data (Li et al., 2007), Huperzine A’s β-elimination half-life is approximately 716 minutes (~12 hours). With daily dosing, plasma concentrations do not return to baseline between doses. Accumulation occurs progressively. Over 2–4 weeks of daily use, the degree of AChE inhibition becomes continuous rather than episodic, the cholinergic system undergoes compensatory downregulation, and marginal cognitive benefit diminishes while side effect risk — particularly insomnia and GI disturbance — increases.
Cycling protocols that work well in practice:
- Standard cycling: 4–6 weeks on, 1–2 weeks off — appropriate for most healthy users
- Conservative cycling: 5 days on, 2 days off — suitable for those with higher sensitivity or longer-term protocols
- No continuous use: Extended uninterrupted use without breaks is not appropriate regardless of dose
The cycling requirement is not theoretical — it is the direct pharmacological consequence of a long-half-life AChE inhibitor. Every pharmaceutical AChE inhibitor used in Alzheimer’s treatment is monitored clinically for tolerance development. Huperzine A is not exempt from this pharmacology simply because it is sold as a supplement.
Timing and Onset: What to Expect and When
The Li et al. (2007) pharmacokinetic study in 12 healthy volunteers (0.4 mg oral dose) found the compound appeared in plasma within 5–10 minutes, reached peak concentration at approximately 58 minutes (Tmax), and showed a biphasic elimination pattern: rapid initial distribution (α half-life ~21 minutes) followed by slow terminal elimination (β half-life ~716 minutes, or approximately 12 hours).
In practice, users typically report the onset of effects — increased mental clarity, slightly heightened verbal fluency, improved sequential reasoning — within 45–90 minutes of dosing. The effect profile is notably different from stimulant nootropics: there is no cardiovascular activation, no anxiogenic edge, and no obvious peak-and-crash pattern. Cholinergic enhancement tends to feel quieter and more cognitive than adrenergic enhancement — improved word retrieval, easier recall under cognitive load, better retention of material encountered during the active window.
Unlike Bacopa Monnieri (which requires 8–12 weeks of accumulation before effects become apparent) or Magnesium L-Threonate (which requires 4–6 weeks minimum), Huperzine A produces acute effects from the first dose — consistent with its mechanism: AChE inhibition begins immediately upon absorption.
Huperzine A is best taken in the morning or early afternoon. Evening dosing is associated with reports of insomnia and vivid dreaming, consistent with the known effect of elevated acetylcholine during sleep on REM dream vividness and cholinergic arousal pathways.
Safety, Side Effects, and Drug Interactions
Huperzine A has a favorable safety record in clinical trials — but “favorable” is not the same as “unremarkable.” Its side effect profile is entirely consistent with its mechanism: all significant adverse effects are cholinergic in nature.
Common Side Effects
At standard supplementation doses (50–100 mcg), side effects are uncommon in healthy adults. At higher doses or with accumulation from un-cycled daily use, the following may occur: nausea, vomiting, diarrhea, dizziness, headache, insomnia, excessive dreaming, slight muscle twitching, increased salivation, and sweating. These are all direct consequences of excess cholinergic activity and resolve when the compound clears the system.
Bradycardia (slowed heart rate) is a specific concern worth noting independently. The cholinergic nervous system regulates cardiac rhythm through the vagus nerve, and AChE inhibition can produce measurable heart rate reduction — a well-documented effect of pharmaceutical AChE inhibitors. This is particularly relevant for individuals with pre-existing bradycardia, cardiac conduction abnormalities, or those taking beta-blockers.
Drug Interactions: The Non-Negotiable Contraindication
The most important safety consideration is Huperzine A’s interaction with prescription cholinesterase inhibitor medications. If you or anyone you recommend this compound to is currently taking donepezil (Aricept), rivastigmine (Exelon), or galantamine (Razadyne), Huperzine A is contraindicated. Combining two AChE inhibitors creates additive or synergistic acetylcholine accumulation that can trigger a cholinergic crisis: severe nausea, vomiting, bradycardia, excessive secretions, muscle weakness, and in extreme cases, respiratory compromise.
This is not a theoretical concern to be mentioned in small print. For a NeuroEdge Formula audience that skews toward adults over 45, many of whom have parents or family members on these medications, this interaction needs to be explicitly understood before any recommendation is made to others.
Additional interactions: beta-blockers (additive bradycardia risk), anticonvulsant medications (limited data; caution warranted), and any compound with cholinergic agonist activity.
General Safety Profile
Toxicology studies show Huperzine A to be non-toxic even when administered at 50–100 times the human therapeutic dose, and the compound has been used by over 100,000 clinical patients in China without reported serious adverse events at standard doses. Huperzine A is not recommended during pregnancy or lactation due to insufficient safety data for these populations.
Build Your Complete Cholinergic Stack
The Alpha-GPC + Huperzine A combination is just one layer of an optimized cognitive stack. Our 7-day protocol PDF walks through how to sequence and time multiple compounds — including this cholinergic triad — within a complete daily protocol.
Stack Integration: The Cholinergic Triad and Beyond
Huperzine A’s most powerful application is as the third component in the cholinergic triad: Alpha-GPC (build) + Phosphatidylserine (release) + Huperzine A (protect).
The Alpha-GPC + Huperzine A Core Stack
This is one of the most mechanistically well-supported nootropic combinations available. Alpha-GPC’s rapid uptake (Tmax ~1 hour) precedes Huperzine A’s peak (Tmax ~60 minutes), creating a coordinated cholinergic window where substrate arrives and AChE inhibition follows closely to extend the resulting acetylcholine signal. Clinical pharmacology supports a starting ratio of 300 mg Alpha-GPC to 50 mcg Huperzine A, taken together in the morning — enough choline substrate for meaningful ACh synthesis, with enough AChE inhibition to extend the signal without pushing into cholinergic excess.
Because both compounds have relatively long activity windows (Alpha-GPC sustains elevated choline for 8–10 hours; Huperzine A inhibits AChE for 10–14 hours), once-daily morning dosing covers the full working day without a second dose.
Adding Phosphatidylserine: The Full Triad
Phosphatidylserine completes the triad by supporting the presynaptic membrane environment that facilitates ACh release. You are now addressing three distinct points in the acetylcholine pathway: substrate availability (Alpha-GPC), release facilitation (PS), and enzymatic breakdown prevention (Huperzine A). Whether this three-way combination produces effects meaningfully greater than the two-compound stack has not been directly studied, but the mechanistic rationale is coherent and the compounds have no known interactions with each other.
A morning protocol combining 300 mg Alpha-GPC + 100 mg Phosphatidylserine + 50–100 mcg Huperzine A represents a reasonable cholinergic foundation for a cognitive performance stack.
Huperzine A and Lion’s Mane: Complementary Hippocampal Support
Lion’s Mane mushroom works through an entirely different mechanism — stimulating Nerve Growth Factor (NGF) production, which supports the growth and maintenance of cholinergic neurons themselves. Where Huperzine A optimizes the neurotransmitter signal within existing cholinergic synapses, Lion’s Mane supports the health and density of the neuronal infrastructure underlying those synapses. These mechanisms are non-overlapping and theoretically complementary for long-term brain health goals, and there are no known interactions between the two compounds.
What Not to Stack
Do not combine Huperzine A with other AChE-inhibiting compounds — including prescription cholinesterase inhibitors and any other supplement with direct cholinergic agonist activity. The ceiling on cholinergic enhancement is real: beyond a threshold, excess acetylcholine produces cognitive impairment rather than enhancement, a phenomenon documented in both animal and human studies. The cycling requirement applies regardless of what Huperzine A is stacked with.
Frequently Asked Questions
Is Huperzine A the same as a pharmaceutical Alzheimer’s drug?
Mechanistically, yes — it works through the same AChE inhibition pathway as donepezil, rivastigmine, and galantamine. In China, it is an approved prescription drug for Alzheimer’s disease. In the United States, it is classified as a dietary supplement, which means it is legally sold without a prescription but lacks FDA approval as a drug. The pharmacological activity is real regardless of regulatory classification, and it should be treated with the same respect as a pharmaceutical AChE inhibitor when considering dosing, cycling, and drug interactions.
Can I take Huperzine A every day?
No. Daily continuous use is not appropriate due to accumulation from the ~12-hour β-elimination half-life, which prevents plasma levels from returning to baseline between doses. Standard guidance is 4–8 weeks of use followed by 1–2 weeks off. The off-cycle period allows AChE activity to normalize and prevents the receptor desensitization that makes extended continuous use both less effective and potentially more problematic.
How does Huperzine A compare to Alpha-GPC for memory?
They address different points in the same pathway and are better understood as complementary than competitive. Alpha-GPC increases acetylcholine supply by providing choline substrate upstream of synthesis. Huperzine A reduces acetylcholine destruction by inhibiting the enzyme that breaks it down. Together, they affect both the production and the duration of the neurotransmitter signal. Neither is a substitute for the other; the most evidence-supported approach uses both at appropriate doses.
Is Huperzine A safe for long-term use?
The available evidence from Chinese clinical programs — over 100,000 patients without reported serious adverse events — is reassuring. However, long-term safety data specifically in healthy adult nootropic users is genuinely limited. Using it in structured on-off cycles at conservative doses (50–100 mcg) rather than continuously at maximum dose is the appropriate risk management approach given current evidence.
What does Huperzine A actually feel like?
The subjective experience is quieter than stimulant-based nootropics. Most users report improved word retrieval and verbal fluency, slightly easier sequential reasoning and working memory under load, and better retention of material studied during the active window. There is no cardiovascular activation, no mood elevation, and no obvious high. The onset is gradual — typically 45–90 minutes, consistent with the Tmax of ~58 minutes documented in pharmacokinetic studies. Cholinergic enhancement sharpens the machinery; it does not add fuel to the engine.
Scientific References
- Yang G, Wang Y, Tian J, Liu JP. Huperzine A for Alzheimer’s disease: a systematic review and meta-analysis of randomized clinical trials. PLOS ONE. 2013;8(9):e74916.
- Xing SH, Zhu CX, Zhang R, An L. Huperzine A in the treatment of Alzheimer’s disease and vascular dementia: a meta-analysis. Evid Based Complement Alternat Med. 2014;2014:363985.
- Wang BS, Wang H, Wei ZH, et al. Efficacy and safety of natural acetylcholinesterase inhibitor huperzine A in the treatment of Alzheimer’s disease: an updated meta-analysis. J Neural Transm. 2009;116(4):457–465.
- Li J, Wu HM, Zhou RL, Liu GJ, Dong BR. Huperzine A for mild cognitive impairment (Cochrane Review). Cochrane Database Syst Rev. 2008;(2):CD005592.
- Rafii MS, Walsh S, Little JT, et al. A phase II trial of huperzine A in mild to moderate Alzheimer disease. Neurology. 2011;76(16):1389–1394.
- Li YX, Zhang RQ, Li CR, Jiang XH. Pharmacokinetics of huperzine A following oral administration to human volunteers. Eur J Drug Metab Pharmacokinet. 2007;32(4):183–187.
- Wessinger CM, Inman CL, Weinstock J, Weiss EP. Effect of Huperzine A on cognitive function and perception of effort during exercise: a randomized double-blind crossover trial. Int J Exerc Sci. 2021;14(2):727–741.
- Zheng W, Xiang YQ, Li XB, et al. Adjunctive huperzine A for cognitive deficits in schizophrenia: a systematic review and meta-analysis. Hum Psychopharmacol. 2016;31(4):286–295.
- Cheng DH, Ren H, Tang XC. Huperzine A, a novel promising acetylcholinesterase inhibitor. Neuroreport. 1996;8(1):97–101.
- Qian BC, Wang M, Zhou ZF, Chen K, Zhou RR, Chen GS. Pharmacokinetics of tablet huperzine A in six volunteers. Acta Pharmacol Sin. 1995;16(5):396–398.